In PerspectiveLiability Landscape
| inperspective LIABILITY landscape Are your residents safe and sound when in bed? |
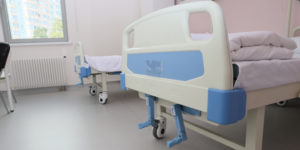
| On August 23, 1995, the U.S. Food and Drug Administration (FDA) issued a Safety Alert to healthcare providers in hospital, nursing home, home health, and hospice settings concerning entrapment hazards associated with the use of bed siderails. Included in the advisement were recommendations to prevent such hazards. While this alert helped many facilities provide a safer environment for residents, the FDA continues to receive reports of head and body entrapment incidents involving siderails each year. Recently, a nursing home in the Midwest received a deficiency tag during its annual state survey for having 54 resident beds with either loose siderails or mattresses that were too small for the bed frames. It is clear that the danger of entrapment with siderails still exists in some healthcare settings. Please take the time to review the circumstances surrounding this recent case and make changes as appropriate at your facility. The Situation While troublesome, these behaviors never resulted in any physical injury, and he would always calm down afterward. For his protection, the man’s mother personally selected a previously used electronic bed with half-rails for her son. The mattress appeared to be the right size for the frame, until the man lay on it, leaving a small gap between the mattress and the bedrail. For the next two years, the man’s mother was quite pleased with the care that her son received at the nursing home. One day, though, a staff member walked into the man’s room and found him motionless with his head caught between the half-rail and mattress. The staff member immediately summoned help and initiated cardiopulmonary resuscitation (CPR) in an attempt to revive him. The ambulance personnel transported the man to the hospital, where he was placed on a respirator for a few days until his mother requested that it be discontinued. The man died shortly thereafter, and his death certificate stated that the cause of his death was asphyxiation. The state long-term care survey agency investigated the incident and cited the facility for not having provided a wider mattress for the bed, as well as padding for the half-rail, because he could have been injured numerous times earlier while thrashing about. The man’s distraught mother hired an attorney and sued the nursing home, alleging negligence contributed to her son’s wrongful death. The nursing home’s state survey was permitted to be entered as evidence in support of the plaintiff’s allegation. Although the small gap between the mattress and half-rail had existed for two years, nobody had seemed to notice it or become aware of the danger it imposed. Even the man’s attending physician, who had viewed his bed and half-rail on numerous occasions during examinations, had not perceived the danger. Because the mattress appeared to come with the bed, the facility’s attorney tried to contact the bed’s manufacturer but learned that the bed, while in good working condition, was an older model, and the manufacturer had gone out of business years prior. The lawsuit demand was for just under a million dollars and was settled months later by the nursing home for $590,000. Protecting Your Residents and Facility
Elderly populations are at highest risk for entrapment, especially those with pre-existing conditions, such as confusion, restlessness, lack of muscle control, or a combination of these factors. It is important to always assess a resident’s needs before using side-rails on a regular basis. If the use of siderails is determined to be appropriate, beneficial, and safe for the resident, follow these FDA recommendations:
Be aware that, even if the bed frame and mattress appear safe, sometimes a resident’s limbs can become caught in small siderail openings, causing traumatic injuries. To minimize this danger, assess the resident, discuss options with responsible parties, and plan the resident’s care accordingly. There are many types of bed bolsters, guards, and padding available through various medical supply outlets. Make sure the selected device is always secured and eliminates gaps between rails and mattress. Proactive planning is essential in order to adequately safeguard your residents from potential dangers such as those caused by entrapment hazards described in this case. For more information about bed safety, see the FDA’s Web site at www.fda.gov/cdrh/beds. Finally, the Safe Medical Devices Act of 1990 (SMDA) requires healthcare facilities to report deaths, serious illnesses, and injuries associated with the use of medical devices. Healthcare workers should follow the procedures established in their facility for such mandatory reporting. |
| Linda Williams, RN, is a Long-Term Care Risk Manager for the GuideOne Center for Risk Management’s Senior Living Communities Division. She previously served as Director of Nursing in a CCRC and as a nurse consultant for two corporations with numerous long-term care facilities in Iowa. The GuideOne Center for Risk Management is dedicated to helping churches, senior living communities, and schools/colleges safeguard their communities by providing practical and timely training and resources on safety, security, and risk-management issues. For more information, contact Williams at (877) 448-4331, ext. 5175, or slc@guideone.com, or visit www.guideonecenter.com. To comment on this article, please send an e-mail to williams0604@nursinghomesmagazine.com. For reprints in quantities of 100 or more, call (866) 377-6454. |
Related Articles
Topics: Articles , Facility management , Risk Management